- Biomedical Application of the Horseshoe Crab
- What is Endotoxin?
- Horseshoe Crabs & Endotoxin Testing
- Endotoxin Testing Methods & Regulations
- Alternative Endotoxin Testing Methods
- Endotoxin Timeline
- Endotoxin Testing Manufacturers & Conservation
- Best Manufacturing Practices
- Achieving Sustainability
- The Consumer Role in Conservation
- Sustainable and Responsible Purchasers
Help us save the horseshoe crab...
make a donation now!
Achieving Sustainability
As the human population grows and ages, the need for injectable and implantable pharmaceuticals and medical devices and dialysis products expands as well. This means an increase in the need for LAL and TAL to help assure the safety of these products. LAL and TAL manufacturers can only keep up with the ever increasing demand for LAL and TAL by bleeding more crabs each year.
Increased LAL and TAL production is not sustainable
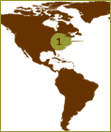
The Atlantic Horseshoe
crab, found primarily
along the eastern U.S
seaboard, is used to
produce LAL.
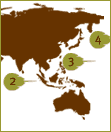
There are three Asian
species; the two
Tachypleus species (3,4)
are used
to make TAL
LAL Production
In the United States, the LAL producers are currently at the upper limits of their harvesting allocation. Up until now, LAL producers have been able to manage growth through improved harvesting, handling and release protocols, as well as advancements in manufacturing and testing methodologies. However, with production waste at a minimum, and testing methodologies already fine tuned, LAL producers will need to harvest more animals in order to keep pace with global demand.
Harvesting restrictions have helped to stabilize the population of the Atlantic Horseshoe Crab, Limulus polyphemus in the southern and mid-Atlantic regions of its range. However the population is still declining throughout the New England states (see www.asmfc.org, managed species, horseshoe crabs for more information). As we close in on the sustainable harvest capacity of Limulus polyphemus throughout its range, it becomes more difficult to envision how the endotoxins detection industry will meet future global demands, without offsetting their needs against another user group i.e., the conch and eel fishery.
Conservation status of the American horseshoe crab, (Limulus polyphemus): a regional assessment
TAL Production
In Asia, home to 2/3rd of the world’s human population, the three Asian species of horseshoe crabs are in decline. Loss of habitat and human consumption are major factors, as are the methods-used and number of horseshoe crabs collected for the endotoxin detection industry. TAL, which is derived from the two Tachypleus species, is only produced in China. With all indicators pointing to a decline in the population, Tachypleus cannot continue to support TAL production for the Asian pharmaceutical and medical device industries. While there are no reliable harvesting figures for China's TAL industry, we know the horseshoe crabs are generally harvested in pairs, bled to death and sold to secondary markets for human consumption and chitin, resulting in a 100% mortality. The dramatic decline of Tachypleus along the coast of China has driven harvesters into neighboring countries, exploiting Tachypleus where regulations and enforcement policies are lax. This unsustainable practice, if not abated, will not only decimate the remaining horseshoe population in China, but also more rapidly reduce the Tachypleus population throughout SE Asia, where it is already struggling to survive.
Once TAL is no longer available, there will be an even greater demand for LAL.
The need for alternative methods
Alternative endotoxin detection methods and sustainable resource management practices are needed in order to assure the continuation of a reliable supply of injectable and implantable pharmaceuticals and medical devices. Alternative methods are currently available and more are in development. The pharmaceutical and medical device industry can protect the long-term availability of endotoxin testing products and become partners in the conservation of the world’s four horseshoe crab species by utilizing supply chain initiatives that integrate social and environmental considerations into the qualification and choice of their vendors.
A simple way for these industries to advance horseshoe crab conservation is to adopt alternative methods for their raw material and in-process testing programs. By reducing their demand for LAL and TAL, they are also ensuring the reliability and sustainability of their supply chain for this critical quality control test.
The horseshoe crab-based LAL and TAL methods have, for the most part, replaced the pyrogen test that used rabbits. It is time to retire the horseshoe crab from endotoxin testing. Sustainable alternative methods to the LAL and TAL tests have been developed and are becoming increasingly accepted by regulatory agencies and the industry. This will assure that we have the products we need for our health and the world’s four horseshoe crab species will continue their epic journey through time.
Learn more
ERDG’s peer reviewed paper on how the harvesting of Asian horseshoe crabs for endotoxin detection is not sustainable.
Current Horseshoe Crab Harvesting Practices Cannot Support Global Demand for TAL/ LAL